Atara Biotherapeutics, Inc. (LON:0HIY)
 London · Delayed Price · Currency is GBP · Price in USD
London · Delayed Price · Currency is GBP · Price in USD | Market Cap | 25.85M -19.8% |
| Revenue (ttm) | 113.03M +51.3% |
| Net Income | 17.41M |
| EPS | 1.46 |
| Shares Out | n/a |
| PE Ratio | 1.49 |
| Forward PE | 2.02 |
| Dividend | n/a |
| Ex-Dividend Date | n/a |
| Volume | 2,815 |
| Average Volume | 38,411 |
| Open | 4.850 |
| Previous Close | 4.630 |
| Day's Range | 4.580 - 4.930 |
| 52-Week Range | 4.200 - 19.050 |
| Beta | -0.40 |
| RSI | 26.97 |
| Earnings Date | Feb 6, 2026 |
About Atara Biotherapeutics
Atara Biotherapeutics, Inc. engages in the development of transformative therapies for patients with cancer and autoimmune disease in the United States and the United Kingdom. Its lead product includes Tab-cel (tabelecleucel), a T-cell immunotherapy program that is in Phase 3 clinical trials for the treatment of epstein-barr virus (EBV) with post-transplant lymphoproliferative disease, as well as other EBV-driven diseases. The company has research collaboration agreements with Memorial Sloan Kettering Cancer Center, and Council of the Queenslan... [Read more]
Financial Performance
In 2024, Atara Biotherapeutics's revenue was $128.94 million, an increase of 1404.02% compared to the previous year's $8.57 million. Losses were -$85.40 million, -69.07% less than in 2023.
Financial numbers in USD Financial StatementsNews

INVESTOR ALERT: Pomerantz Law Firm Investigates Claims on Behalf of Investors of Atara Biotherapeutics, Inc. - ATRA
NEW YORK, Jan. 27, 2026 (GLOBE NEWSWIRE) -- Pomerantz LLP is investigating claims on behalf of investors of Atara Biotherapeutics, Inc. (“Atara” or the “Company”) (NASDAQ: ATRA). Such investors are a...

INVESTOR ALERT: Pomerantz Law Firm Investigates Claims On Behalf of Investors of Atara Biotherapeutics, Inc. - ATRA
NEW YORK, Jan. 22, 2026 /PRNewswire/ -- Pomerantz LLP is investigating claims on behalf of investors of Atara Biotherapeutics, Inc. ("Atara" or the "Company") (NASDAQ: ATRA). Such investors are advis...

INVESTOR ALERT: Pomerantz Law Firm Investigates Claims On Behalf of Investors of Atara Biotherapeutics, Inc. - ATRA
NEW YORK, Jan. 20, 2026 (GLOBE NEWSWIRE) -- Pomerantz LLP is investigating claims on behalf of investors of Atara Biotherapeutics, Inc. (“Atara” or the “Company”) (NASDAQ: ATRA). Such investors are ad...

Atara's Outlook As Ebvallo Hits Second FDA CRL
Manufacturing Fixed, But Trial Interpretability Now Blocks US Approval Drug & Indication Overview Atara Biotherapeutics (NASDAQ: ATRA) has developed Ebvallo (tabelecleucel), a novel second-line immun...
Canaccord Genuity Downgrades Atara Biotherapeutics (ATRA) to Hold | ATRA Stock News
Canaccord Genuity Downgrades Atara Biotherapeutics (ATRA) to Hold | ATRA Stock News
Atara Biotherapeutics Stock Falls After FDA CRL On EBVALLO BLA
(RTTNews) - Atara Biotherapeutics Inc. (ATRA) announced that the U.S. Food and Drug Administration issued a Complete Response Letter (CRL) for the Biologics License Application (BLA) of EBVALLO (tabel...
Atara Biotherapeutics (ATRA) Shares Plunge After FDA Setback
Atara Biotherapeutics (ATRA) Shares Plunge After FDA Setback
Monday Sector Laggards: Biotechnology, Credit Services & Lending Stocks
In trading on Monday, biotechnology shares were relative laggards, down on the day by about 2.2%. Helping drag down the group were shares of Atara Biotherapeutics, off about 54% and shares of Prelude ...

FDA Rejects Atara Biotherapeutics' Cell Therapy Again, Stock Sinks
Atara Biotherapeutics stock drops on FDA ... Full story available on Benzinga.com
FDA Rejects Atara's (ATRA) Ebvallo BLA: Calls for New Evidence on Effectiveness
FDA Rejects Atara's (ATRA) Ebvallo BLA: Calls for New Evidence on Effectiveness

Atara Biotherapeutics Provides Regulatory and Business Update on EBVALLO™ (tabelecleucel)
THOUSAND OAKS, Calif.--(BUSINESS WIRE)--Atara Biotherapeutics, Inc. (Nasdaq: ATRA), a leader in T-cell immunotherapy, leveraging its novel allogeneic Epstein-Barr virus (EBV) T-cell platform to develo...
Atara Biotherapeutics (ATRA) Sees Price Target Increase by Canaccord Genuity | ATRA Stock News
Atara Biotherapeutics (ATRA) Sees Price Target Increase by Canaccord Genuity | ATRA Stock News

Atara Biotherapeutics: An Empty Pipeline With Little Gas
Atara nears an FDA-backed cell therapy milestone with no commercialization rights and a suspended pipeline offering few catalysts. Learn why ATRA stock is a sell.
Atara Biotherapeutics (ATRA) Reduces Workforce by 29% in Strategic Move
Atara Biotherapeutics (ATRA) Reduces Workforce by 29% in Strategic Move
Atara Biotherapeutics (ATRA) Exceeds Q3 Revenue Estimates
Atara Biotherapeutics (ATRA) Exceeds Q3 Revenue Estimates

Atara Biotherapeutics Announces Third Quarter Financial Results and Operational Progress
THOUSAND OAKS, Calif.--(BUSINESS WIRE)--Atara Biotherapeutics, Inc. (Nasdaq: ATRA), a leader in T-cell immunotherapy, leveraging its novel allogeneic Epstein-Barr virus (EBV) T-cell platform to develo...
Atara Biotherapeutics (ATRA) Surges 10.0%: Is This an Indication of Further Gains?
Atara Biotherapeutics (ATRA) witnessed a jump in share price last session on above-average trading volume. The latest trend in earnings estimate revisions for the stock doesn't suggest further strengt...
Atara Biotherapeutics (ATRA) Transfers Key FDA Application to Pierre Fabre
Atara Biotherapeutics (ATRA) Transfers Key FDA Application to Pierre Fabre

Pierre Fabre Pharmaceuticals Announces Transfer from Atara Biotherapeutics of the Biologics License Application (BLA) for Tabelecleucel as Treatment of Epstein-Barr Virus Positive Post-Transplant Lymphoproliferative Disease (EBV+ PTLD)
Tabelecleucel BLA currently under U.S. Food and Drug Administration (FDA) Priority Review as potentially the first approved therapy in the U.S. for EBV+ PTLD with a Prescription Drug User Fee Act (PDU...
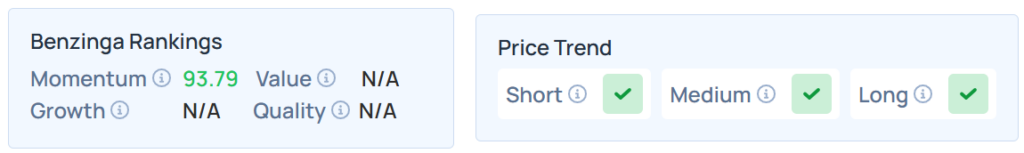
4 Biotechnology Stocks That Outshine In Momentum Amid Strong Technicals
Four biotechnology stocks have surged into the top 10th percentile for momentum ranking, demonstrating remarkable week-on-week technical strength and strong price action. Spotlight On 4 Biotech Stocks...

4 Biotechnology Stocks That Outshine In Momentum Amid Strong Technicals
Four biotechnology stocks have surged into the top 10th percentile for momentum ranking, demonstrating remarkable week-on-week technical strength and strong price action.
Atara Biotherapeutics (ATRA) Soars 10.0%: Is Further Upside Left in the Stock?
Atara Biotherapeutics (ATRA) saw its shares surge in the last session with trading volume being higher than average. The latest trend in earnings estimate revisions may not translate into further pric...

Atara Biotherapeutics Announces Changes to Its Board of Directors
THOUSAND OAKS, Calif--(BUSINESS WIRE)--Atara Biotherapeutics, Inc. (Nasdaq: ATRA), a leader in T-cell immunotherapy, leveraging its novel allogeneic Epstein-Barr virus (EBV) T-cell platform to develop...

Atara Biotherapeutics Announces Second Quarter Financial Results and Operational Progress
THOUSAND OAKS, Calif.--(BUSINESS WIRE)--Atara Biotherapeutics, Inc. (Nasdaq: ATRA), a leader in T-cell immunotherapy, leveraging its novel allogeneic Epstein-Barr virus (EBV) T-cell platform to develo...

Pierre Fabre Pharmaceuticals Inc. Announces FDA Acceptance and Priority Review of the Biologics License Application (BLA) for Tabelecleucel for the Treatment of Epstein-Barr Virus Positive Post-Transplant Lymphoproliferative Disease (EBV+ PTLD)
First allogeneic T-Cell therapy BLA offers hope to EBV+ PTLD patients who have limited treatment options and lifespan measured in only a few weeks to months following failure of initial treatment EBV+...