Genmab A/S (CPH:GMAB)
 Denmark · Delayed Price · Currency is DKK
Denmark · Delayed Price · Currency is DKK | Market Cap | 112.95B +30.0% |
| Revenue (ttm) | 23.66B +19.2% |
| Net Income | 6.13B -15.0% |
| EPS | 97.77 -12.3% |
| Shares Out | 61.57M |
| PE Ratio | 18.76 |
| Forward PE | 23.82 |
| Dividend | n/a |
| Ex-Dividend Date | n/a |
| Volume | 26,866 |
| Average Volume | 126,971 |
| Open | 1,852.00 |
| Previous Close | 1,834.50 |
| Day's Range | 1,840.00 - 1,869.00 |
| 52-Week Range | 1,157.00 - 2,257.00 |
| Beta | 0.80 |
| RSI | 42.24 |
| Earnings Date | Feb 17, 2026 |
About Genmab
Genmab A/S, a biotechnology company, develops antibody-based products and product candidates for the treatment of cancer and other diseases in Denmark. The company markets EPKINLY and TEPKINLY for adult patients with relapsed or refractory (R/R) diffuse large b-cell lymphoma (DLBCL), large B-cell lymphoma, and follicular lymphoma (FL); and Tivdak for adult patients with recurrent/metastatic cervical cancer with disease progression on or after chemotherapy. It is also developing Epcoritamab for R/R DLBCL and FL, first line DLBCL and FL, B-cell n... [Read more]
Financial Performance
In 2025, Genmab's revenue was $3.72 billion, an increase of 19.19% compared to the previous year's $3.12 billion. Earnings were $963.00 million, a decrease of -15.00%.
Financial numbers in USD Financial StatementsNews

Genmab to Participate in Upcoming Investor Conferences
Media Release COPENHAGEN, Denmark; February 25, 2026 Genmab A/S (Nasdaq: GMAB) announced today that members of its E xecutive Committee will participat e in fireside chats at the following investor c...

Capital Increase in Genmab as a Result of Employee Warrant Exercise
Company Announcement COPENHAGEN, Denmark; February 24, 2026 – Genmab A/S (Nasdaq: GMAB) will increase its share capital by 12,313 shares as a consequence of the exercise of employee warrants. The incr...

Transactions in Connection with Share Buy-back Program
Company Announcement COPENHAGEN, Denmark; February 23, 2026 – Genmab A/S (Nasdaq: GMAB). On February 17, 2026, Genmab announced the initiation of a share buy-back program to repurchase up to 342,130 s...

Transactions with shares and linked securities in Genmab A/S made by managerial employees and their closely associated persons
Company Announcement COPENHAGEN, Denmark; February 20, 2026 – Genmab A/S (Nasdaq: GMAB) - In accordance with Article 19 of Regulation No. 596/2014 on Market Abuse and Implementing Regulation 2016/523,...

Novo Nordisk nominates three new board directors, two with pharma experience
Novo Nordisk new board nominations: Genmab co-founder Jan van de Winkel, ex-Takeda/Eli Lilly’s Ramona Sequeira and finance expert Helena Saxon. Read more here.
Top 3 Health Care stocks That Could Lead To Your Biggest Gains In Q1
The most oversold stocks in the health care sector presents an opportunity to buy into undervalued companies.

Notice to Convene the Annual General Meeting of Genmab A/S
Company Announcement Genmab A/S to hold Annual General Meeting on Thursday March 19, 2026 COPENHAGEN, Denmark; February 18, 2026 – Genmab A/S (Nasdaq: GMAB) summons the Annual General Meeting on Thurs...
Genmab GAAP EPS of $15.37, revenue of $3.72B misses by $50M; initiates FY26 outlook
Genmab AS (GMAB) Q4 2025 Earnings Call Highlights: Strong Revenue Growth and Strategic Expansions
Genmab AS (GMAB) Q4 2025 Earnings Call Highlights: Strong Revenue Growth and Strategic Expansions
Q4 2025 Genmab A/S Earnings Call Transcript
Q4 2025 Genmab A/S Earnings Call Transcript
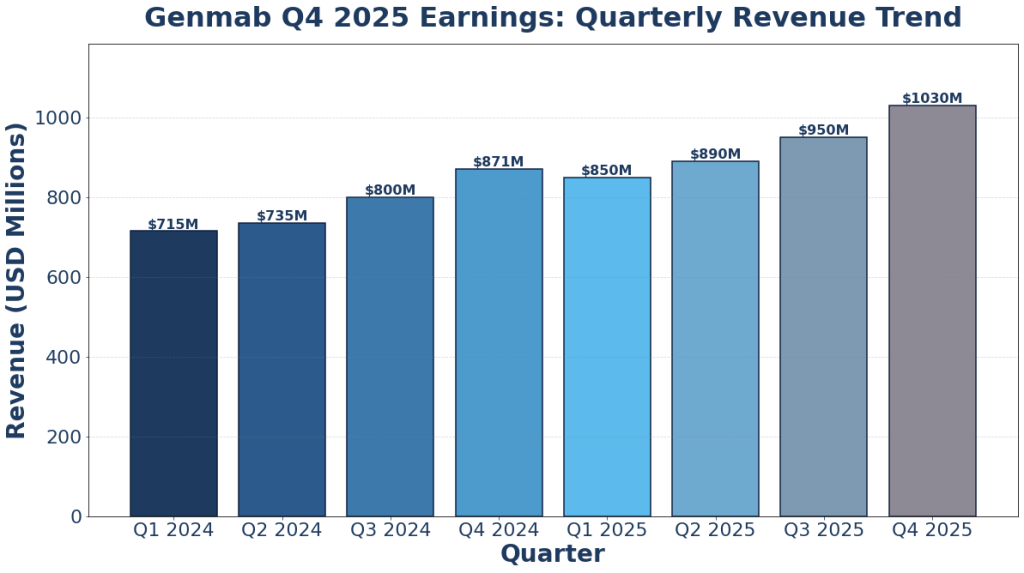
Genmab Q4 2025 Earnings Report: Innovation Push Drives Profit Surge
Genmab A/S (Nasdaq: GMAB) released its full-year 2025 financial results, demonstrating the company’s improved operational execution. Total revenue reached $3.72... The post Genmab Q4 2025 Earnings Rep...

Genmab: Q4 Earnings Insights
Genmab (NASDAQ: GMAB) announced its Q4 earnings on Tuesday, February 17, 2026 at 11:00 AM. Here's a breakdown of the earnings report. Earnings Genmab missed estimated earnings by -88.64%, reporting a...
Genmab A/S (GMAB) Q4 2025 Earnings Call Transcript

Genmab Announces Initiation of Share Buy-Back Program
Company Announcement Repurchase of up to 342,130 shares with a maximum aggregate total value of 725 million DKK Honoring our commitments under the Restricted Stock Unit program Completion expected no ...
Genmab A/S 2025 Q4 - Results - Earnings Call Presentation
Genmab Sees FY26 Revenue Of Up To $4.4 Bln
(RTTNews) - Genmab A/S (GMAB) published its 2025 annual report and projected higher revenue for 2026, supported by royalty growth and strong product sales.
Genmab A/S Full Year Profit Drops
BRUSSELS (dpa-AFX) - Genmab A/S (GMAB) released earnings for full year that Dropped, from the same period last yearThe company's bottom line totaled $963 million, or $15.37 per share. This compare...

Genmab Files Annual Report with the U.S. Securities and Exchange Commission
Media Release COPENHAGEN, Denmark; February 17, 2026 Genmab filed Form 20-F for the financial year 2025 with the U.S. SEC Genmab A/S (Nasdaq: GMAB) a nnounced today that it has filed its Annual Repor...

Genmab Publishes 2025 Annual Report
Company Announcement COPENHAGEN, Denmark; February 17, 2026 – Genmab A/S (Nasdaq: GMAB) announced today the publication of its Annual Report for 2025. Below is a summary of business progress in 2025, ...
Genmab (GMAB) Receives Buy Rating from Jefferies with Positive Outlook
Genmab (GMAB) Receives Buy Rating from Jefferies with Positive Outlook

A Peek at Genmab's Future Earnings
Genmab (NASDAQ: GMAB) is set to give its latest quarterly earnings report on Tuesday, 2026-02-17. Here's what investors need to know before the announcement. Analysts estimate that Genmab will report...
Genmab (GMAB) Receives Equal-Weight Rating from Morgan Stanley | GMAB Stock News
Genmab (GMAB) Receives Equal-Weight Rating from Morgan Stanley | GMAB Stock News
Genmab (GMAB) Receives Equal Weight Rating from Morgan Stanley
Genmab (GMAB) Receives Equal Weight Rating from Morgan Stanley
Genmab AS (GMAB) Q4 2025 Earnings Report Preview: What To Expect
Genmab AS (GMAB) Q4 2025 Earnings Report Preview: What To Expect
Pre-Market Earnings Report for February 17, 2026 : MDT, ET, VMC, DTE, LH, LDOS, GPC, SGI, GMAB, CNH, ALLE, WSO
The following companies are expected to report earnings prior to market open on 02/17/2026. Visit our Earnings Calendar for a full list of expected earnings releases.Medtronic plc (MDT)is reporting fo...